Diving into the Complexity of Potocki-Lupski Syndrome: Unraveling the Genetic Landscape and Clinical Implications.
Explore this collection of scientific articles dedicated to shedding light on the multifaceted aspects of PTLS. From its genetic underpinnings to the intricate clinical manifestations, fostering a deeper understanding and paving the way for innovative approaches in potential therapies.
-
![Illustration of a blue DNA double helix on a science-themed background]()
Journal of Neurodevelopmental Disorders
The behavioural phenotype of Potocki-Lupski syndrome: a cross-syndrome comparison
-
![3D illustration of human cells with pink nuclei, surrounded by clear membranes, floating in a blue environment.]()
RAI1 Overexpression
RAI1 Overexpression Promotes Altered Circadian Gene Expression and Dyssomnia in Potocki–Lupski Syndrome
-
![Graph showing Rai1 dosage related to Smith-Magenis and dup(17)(p11.2) syndromes. The x-axis represents different genetic variations and the y-axis indicates Rai1 dosage levels, divided into sections for Rai1+/-, hemizygous transgenics, and homozygous transgenics. Each section is color-coded.]()
European Journal of Human Genetics
How much is too much? Phenotypic consequences of Rai1 overexpression in mice
-
![Composite image showing fluorescent microscopy of brain sections stained with DAPI and eGFP, highlighting various brain regions such as cortex (CX), hippocampus (HC), striatum (ST), and amygdala (AG). Additional panels demonstrate specific staining for neuronal marker NeuN and glial marker GFAP with merged images.]()
Oxford Academic, Human Molecular Genetics
Correct developmental expression level of Rai1 in forebrain neurons is required for control of body weight, activity levels and learning and memory
-
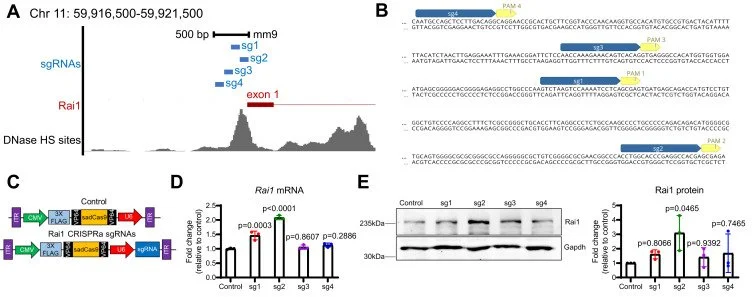
![Scientific illustration showing CRISPR-Cas9 targeting of Rai1 gene. A) Chromosomal location and sgRNA sites of Rai1 gene on Chr 11. B) DNA sequence with sgRNA and PAM sites highlighted. C) Diagram describing experimental setup with Rai1 CRISPRa sgRNAs. D) Bar graph comparing Rai1 mRNA expression levels after sgRNA treatments. E) Western blot and bar graph displaying Rai1 protein levels with control and sgRNA treatments."]()
Journal of biological chemistry
rAAV-CRISPRa therapy corrects Rai1 haploinsufficiency and rescues selective disease features in Smith-Magenis syndrome mice
-
![Diagram of Rai1 genomic modification in mice, microscopy images showing Rai1 expression, and a graph of Rai1 mRNA expression levels over time. Also includes an adult mouse brain section with Rai1-Tag labeling.]()
Science Direct
Molecular and Neural Functions of Rai1, the Causal Gene for Smith-Magenis Syndrome
-
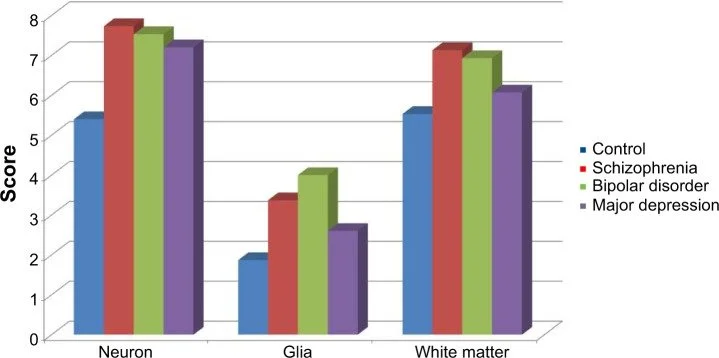
![Bar graph comparing scores of neuron, glia, and white matter across control, schizophrenia, bipolar disorder, and major depression groups, with color-coded data.]()
National Library of Medicine
Increased expression of retinoic acid-induced gene 1 in the dorsolateral prefrontal cortex in schizophrenia, bipolar disorder, and major depression
-
![Illustration of DNA analysis with a double helix model on the left marked with 5' and 3' ends, a magnifying glass indicating focus, and a flat DNA sequence diagram on the right showing base pairs C-G, A-T.]()
Anatomy Of a Gene - Cure rare Disease
Developing gene therapies to treat rare and ultra-rare neuromuscular disorders requires a sound understanding of the anatomy of genes and the various methods by which genetic mutations can be targeted. From our antisense oligonucleotide therapeutic for SCA3 in collaboration with Leiden University Medical Center to our gene replacement strategy for ADSSL1 gene-related myopathy with Boston Children’s Hospital, Cure Rare Disease is employing a variety of techniques to treat genetic diseases.
-
![Business meeting with laptops, papers, and two people collaborating]()
The behavioural phenotype of Potocki-Lupski syndrome: a cross-syndrome comparison
Potocki-Lupski syndrome (PTLS) and Smith-Magenis syndrome (SMS) are related genomic disorders, as duplication 17p11.2 (associated with PTLS) is the reciprocal recombination product of the SMS microdeletion. While SMS has a relatively well-delineated behavioural phenotype, the behavioural profile in PTLS is less well defined, despite purported associations with autism spectrum disorder (ASD) and the suggestion that some behaviours may be diametric to those seen in SMS.
-
![Scientist examining sample under microscope]()
Short stature and growth hormone deficiency in a subset of patients with Potocki–Lupski syndrome: Expanding the phenotype of PTLS
Potocki–Lupski Syndrome (PTLS, MIM 610883), or duplication of chromosome 17p11.2, is a clinically recognizable condition characterized by infantile hypotonia, failure to thrive, developmental delay, intellectual disability, and congenital anomalies. Short stature, classified as greater than two standard deviations below the mean, has not previously been considered a major feature of PTLS. Retrospective chart review on a cohort of 37 individuals with PTLS was performed to investigate the etiology of short stature.
-
![Scientist using pipette for cell culture in a laboratory]()
Comparative analyses of the Smith−Magenis syndrome protein RAI1 in mice and common marmoset monkey
RAI1 is among the top 130 genes strongly associ- ated with idiopathic autism (Trost et al., 2022). This clinical evidence suggests that proper RAI1 levels are essential for brain development and function.
-
![Close-up of a microscope with objective lenses and a sample stage.]()
Variants in TCF20 in neurodevelopmental disability
TCF20 is paralogous to RAI114, the causative gene in Potocki–Lupski syndrome (duplication of 17p11.2), which is associated with autism spectrum disorder (ASD) in ~90% of cases16,17; and Smith–Magenis syndrome (deletion of 17p11.2), characterized by severe intellectual disability and neurobehavioural problems, including ASD
-
![Abstract depiction of DNA strands with a dark background and glowing blue highlights.]()
ToolGen CMT1A Treatment Receives Orphan Drug Designation
TGT-001 corrects genes within the body directly, using the CRISPR gene-editing tool to regulate the expression of PMP22 to normal levels. This treatment is designed to treat CMT1A, which is caused by a duplication of the PMP22 gene.
-
![Scientist operating laboratory equipment with blue gloves]()
Arnold-Chiari type 1 malformation in Potocki-Lupski syndrome
Herein, we describe three patients with PTLS who were found-in the course of routine clinical care-to have a type 1 Arnold-Chiari malformation (CM-1)
-
![Floating human brain on a blue and purple gradient background]()
Correct developmental expression level of Rai1 needed for:
Correct developmental expression level of Rai1 in forebrain neurons is required for control of body weight, activity levels and learning and memory
-
![3D illustration of a transparent human body with DNA helix and molecular structure, highlighting the stomach area with red circles.]()
Definition of a critical genetic interval:
Definition of a critical genetic interval related to kidney abnormalities in Potocki-Lupski syndrome
-
![Close-up of a plasma globe with glowing pink and blue light streams emanating from a central sphere against a dark background.]()
ene Related to Neurobehavioral Alterations
Retinoic Acid Induced 1, RAI1: A Dosage Sensitive Gene Related to Neurobehavioral Alterations Including Autistic Behavior
-
![Person wearing purple gloves holds a tray of labeled test tubes in a lab.]()
Phenotypic consequences of copy number variation:
insights from Smith-Magenis and Potocki-Lupski syndrome mouse models
-
![Complex molecular structure model against a black background]()
Mouse models of genomic syndromes as tools for understanding the basis of complex traits:
An example with the smith-magenis and the potocki-lupski syndromes
-
![Fluorescently stained human cells under a microscope, showing blue nuclei and varying cytoplasmic colors]()
Abnormal social behaviors and altered gene expression rates in a mouse model for Potocki-Lupski syndrome
We have generated a PTLS mouse model, Dp(11)17/+, that recapitulates some of the physical and neurobehavioral phenotypes present in patients.
-
![Close-up of DNA strands with a blue background and particles]()
Rai1 duplication causes physical and behavioral phenotypes in a mouse model of dup(17)(p11.2p11.2)
We previously generated a mouse model for dup(17)(p11.2p11.2), Dp(11)17/+, that recapitulated most of the phenotypes observed in human patients. We have now analyzed compound heterozygous mice carrying a duplication [Dp(11)17] in one chromosome
-
![Fluorescent microscopy image showing cells in blue and pink hues with a central band of concentrated pink cells.]()
Behavioral characterization of mouse models
For Smith-Magenis syndrome and dup(17)(p11.2p11.2)
-
![Orange oil droplets floating in water creating a pattern against a bright orange background.]()
Modeling del(17)(p11.2p11.2) and dup(17)(p11.2p11.2) contiguous gene syndromes
Modeling del(17)(p11.2p11.2) and dup(17)(p11.2p11.2) contiguous gene syndromes by chromosome engineering in mice: phenotypic consequences of gene dosage imbalance
-
![Bar graphs comparing vertical activity, center/total distance ratio, and locomotor activity in four mouse groups: +/+ (black), Dp(11)17/+ (gray), Rai1+/- (dark gray), Dp(11)17/Rai1- (white).]()
Rai1 duplication causes physical and behavioral phenotypes in a mouse model of dup(17)(p11.2p11.2)
We have now analyzed compound heterozygous mice carrying a duplication [Dp(11)17
-
![Close-up of swirling blue and white abstract patterns resembling marble or agate.]()
Evidence for genetic regulation of mRNA expression
Evidence for genetic regulation of mRNA expression of the dosage-sensitive gene retinoic acid induced-1 (RAI1) in human brain -
![Glowing plasma globe with electric strands]()
Retinoic Acid Induced 1, RAI1: A Dosage Sensitive Gene
Retinoic Acid Induced 1, RAI1: A Dosage Sensitive Gene Related to Neurobehavioral Alterations Including Autistic Behavior
-
![Fluorescent microscopy of cells showing blue nuclei, green cytoskeletons, and red extracellular matrix.]()
Molecular cloning and characterization of human RAI1, a gene associated with schizophrenia
The work presented here represents the characterization of the human RAI1 gene. Our results show that this gene is very similar to its mouse ortholog both in DNA and protein sequences and in expression patterns

Work With Us
We are always looking for pharmaceutical companies, medical institutions & potential investors to work with, please get in touch with us and we would love to discuss a collaboration, project or investment further.